Potassium Properties and uses of Potassium The boiling point for potassium is 1425°F Potassium has the melting point of 146°F A slivery color is. - ppt download

Difference Between Sodium and Potassium | Definition, Chemical Properties, Compounds, Isotopes, Similarities and Differences

Potassium Properties and uses of Potassium The boiling point for potassium is 1425°F Potassium has the melting point of 146°F A slivery color is. - ppt download
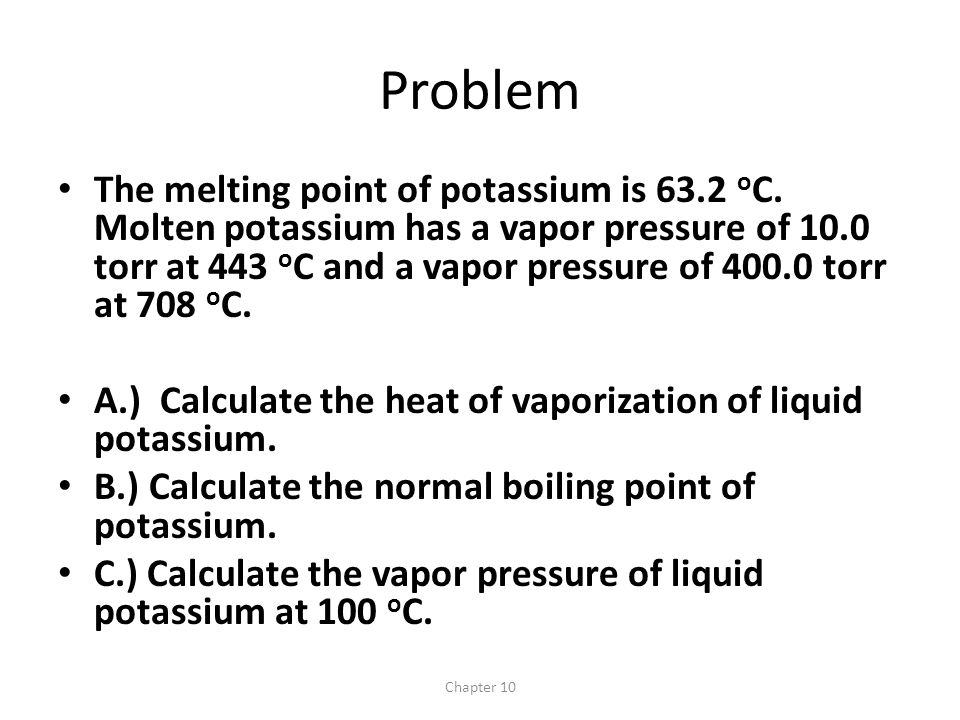
Chapter 10 Problem The melting point of potassium is 63.2 o C. Molten potassium has a vapor pressure of 10.0 torr at 443 o C and a vapor pressure of ppt download

Calculate the boiling point of a 1M aqueous solution (density 1.04 g `Ml^(-1)`) of Potassium - YouTube

Potassium Properties and uses of Potassium The boiling point for potassium is 1425°F Potassium has the melting point of 146°F A slivery color is. - ppt download

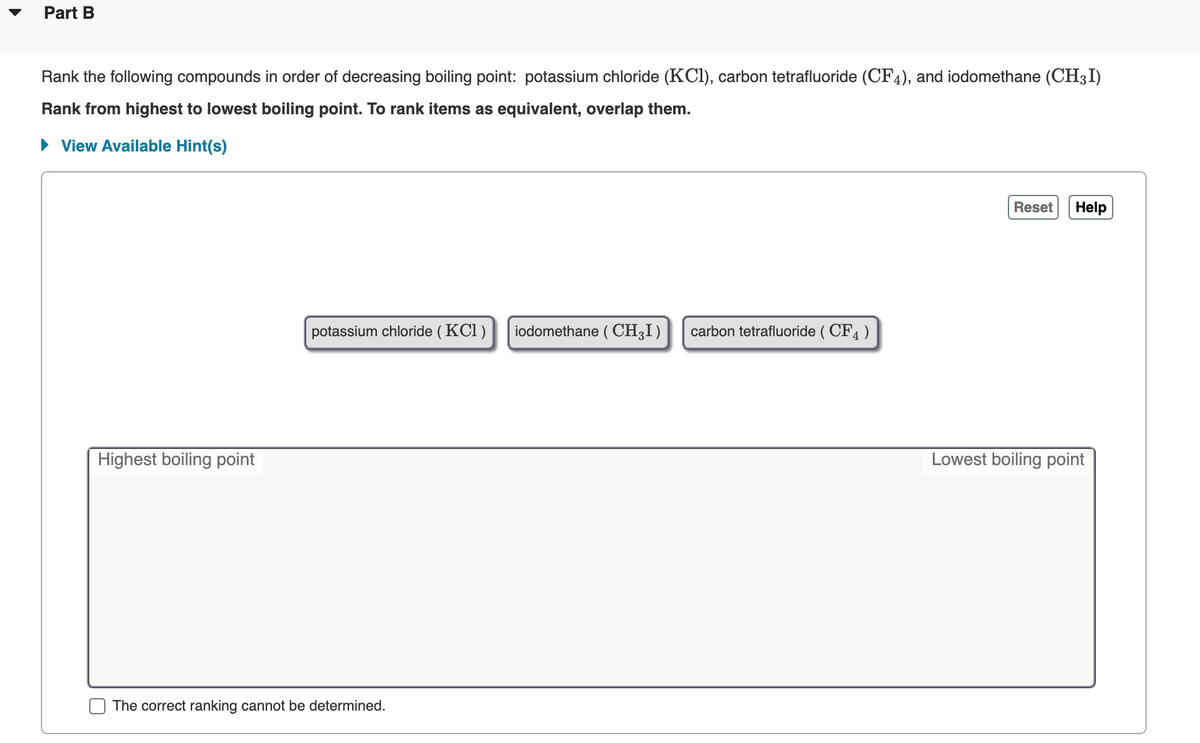
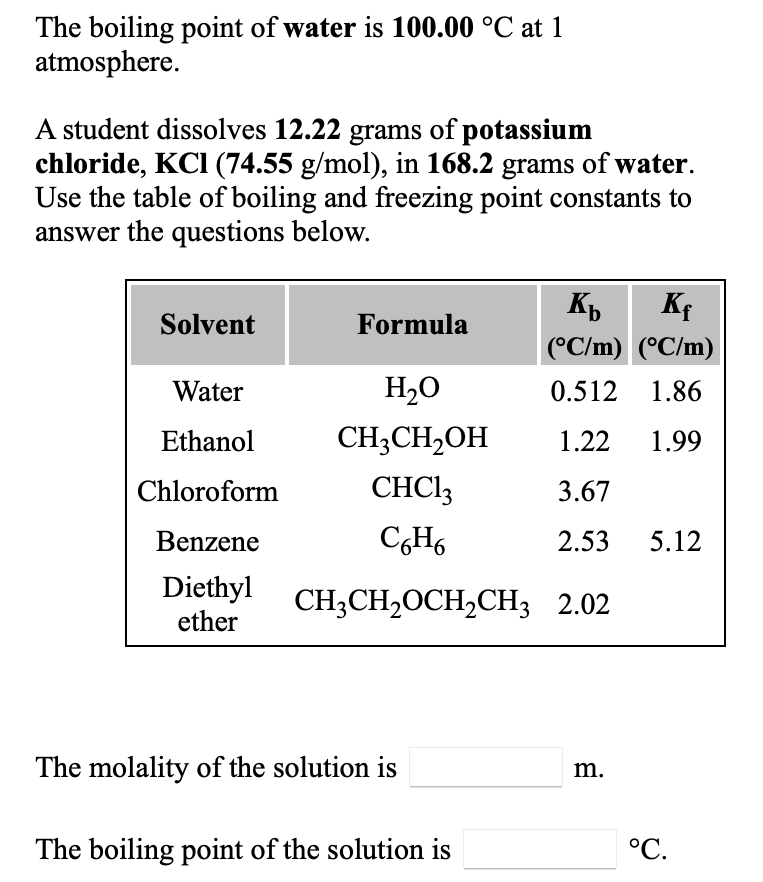
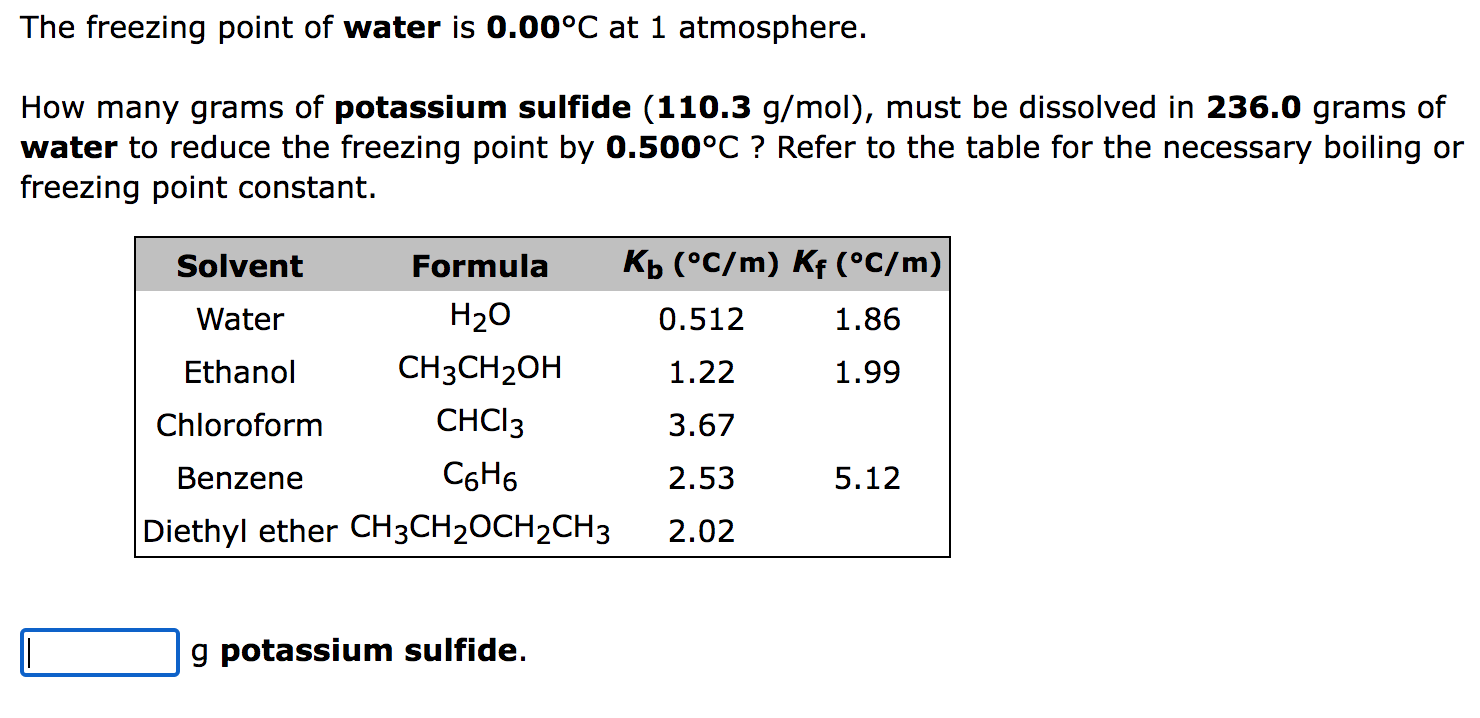
SOLVED: Calculate the boiling point of a solution prepared by dissolving 1ll.9 g of potassium chloride (KCl) in 1000 mL of water? Tb = 0519C mol mol. of particle 100.51 %C 101.02 % 101.53 %C 102.04 %